Kolekce 111+ Atom Geometry Chart
Kolekce 111+ Atom Geometry Chart. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … In the geometry, three atoms are in the same plane with bond angles of 120°; There are lone pairs on x or other atoms, but we don't ca re. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).
Tady Untitled Document
We are interested in only … The other two atoms are on opposite ends of the molecule. Molecular geometry van koppen/offen procedure: Some elements in group 15 of the periodic table form compounds of the type ax 5;Examples include pcl 5 and asf 5.
The other two atoms are on opposite ends of the molecule. Examples include pcl 5 and asf 5. The other two atoms are on opposite ends of the molecule. We are interested in only … 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Increase charge of selected atom +1; Turn off atom manipulation off; It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

In the geometry, three atoms are in the same plane with bond angles of 120°; We are interested in only … Increase charge of selected atom +1; Turn off bond editing off; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. We are interested in only …

Examples include pcl 5 and asf 5.. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. We are interested in only … A = the central atom, x = an atom bonded to a, e = a lone pair on a. The two x atoms (in white) are 180° away from one another. Molecular geometry van koppen/offen procedure: In the geometry, three atoms are in the same plane with bond angles of 120°;.. Turn off bond editing off;

14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … In the geometry, three atoms are in the same plane with bond angles of 120°; Turn off bond editing off; Molecular geometry van koppen/offen procedure: Some elements in group 15 of the periodic table form compounds of the type ax 5;. Increase charge of selected atom +1;

A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule.. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry van koppen/offen procedure: A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Increase charge of selected atom +1; Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). There are lone pairs on x or other atoms, but we don't ca re. The two x atoms (in white) are 180° away from one another. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Turn off bond editing off;

The two x atoms (in white) are 180° away from one another. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … There are lone pairs on x or other atoms, but we don't ca re. A = the central atom, x = an atom bonded to a, e = a lone pair on a. In the geometry, three atoms are in the same plane with bond angles of 120°; 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is ….. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).

A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule.. In the geometry, three atoms are in the same plane with bond angles of 120°; Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Increase charge of selected atom +1; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Examples include pcl 5 and asf 5. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Molecular geometry van koppen/offen procedure: There are lone pairs on x or other atoms, but we don't ca re.

It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … The other two atoms are on opposite ends of the molecule. Molecular geometry van koppen/offen procedure: The two x atoms (in white) are 180° away from one another. We are interested in only … A = the central atom, x = an atom bonded to a, e = a lone pair on a. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …

Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … The other two atoms are on opposite ends of the molecule. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … There are lone pairs on x or other atoms, but we don't ca re. A = the central atom, x = an atom bonded to a, e = a lone pair on a. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Increase charge of selected atom +1; Some elements in group 15 of the periodic table form compounds of the type ax 5; In the geometry, three atoms are in the same plane with bond angles of 120°; Turn off bond editing off; Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …

Turn off bond editing off; The two x atoms (in white) are 180° away from one another. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. The other two atoms are on opposite ends of the molecule. Examples include pcl 5 and asf 5. We are interested in only … A = the central atom, x = an atom bonded to a, e = a lone pair on a. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule... The other two atoms are on opposite ends of the molecule.

The two x atoms (in white) are 180° away from one another. Some elements in group 15 of the periodic table form compounds of the type ax 5; Examples include pcl 5 and asf 5. Increase charge of selected atom +1; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Turn off bond editing off; Molecular geometry van koppen/offen procedure: Turn off atom manipulation off;.. Turn off atom manipulation off;

Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … There are lone pairs on x or other atoms, but we don't ca re. The two x atoms (in white) are 180° away from one another. A = the central atom, x = an atom bonded to a, e = a lone pair on a. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …. We are interested in only …

Increase charge of selected atom +1;.. Some elements in group 15 of the periodic table form compounds of the type ax 5; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. The other two atoms are on opposite ends of the molecule.. In the geometry, three atoms are in the same plane with bond angles of 120°;

A = the central atom, x = an atom bonded to a, e = a lone pair on a.. Turn off bond editing off; We are interested in only … Increase charge of selected atom +1; Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. A = the central atom, x = an atom bonded to a, e = a lone pair on a. In the geometry, three atoms are in the same plane with bond angles of 120°; Some elements in group 15 of the periodic table form compounds of the type ax 5;

Some elements in group 15 of the periodic table form compounds of the type ax 5; . A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule.

We are interested in only …. The other two atoms are on opposite ends of the molecule. A = the central atom, x = an atom bonded to a, e = a lone pair on a. The two x atoms (in white) are 180° away from one another. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … There are lone pairs on x or other atoms, but we don't ca re. Increase charge of selected atom +1; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Some elements in group 15 of the periodic table form compounds of the type ax 5; In the geometry, three atoms are in the same plane with bond angles of 120°;. A = the central atom, x = an atom bonded to a, e = a lone pair on a.

14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Molecular geometry van koppen/offen procedure: Increase charge of selected atom +1; The two x atoms (in white) are 180° away from one another. There are lone pairs on x or other atoms, but we don't ca re. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …

The other two atoms are on opposite ends of the molecule. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … In the geometry, three atoms are in the same plane with bond angles of 120°; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule.

Increase charge of selected atom +1; There are lone pairs on x or other atoms, but we don't ca re.. Molecular geometry van koppen/offen procedure:

We are interested in only …. The other two atoms are on opposite ends of the molecule. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Some elements in group 15 of the periodic table form compounds of the type ax 5;.. Increase charge of selected atom +1;

We are interested in only … 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Some elements in group 15 of the periodic table form compounds of the type ax 5; Turn off atom manipulation off; In the geometry, three atoms are in the same plane with bond angles of 120°; Examples include pcl 5 and asf 5. A = the central atom, x = an atom bonded to a, e = a lone pair on a. The two x atoms (in white) are 180° away from one another. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom)... The other two atoms are on opposite ends of the molecule.

The other two atoms are on opposite ends of the molecule... . We are interested in only …

We are interested in only … A = the central atom, x = an atom bonded to a, e = a lone pair on a. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Some elements in group 15 of the periodic table form compounds of the type ax 5; 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Turn off bond editing off;. We are interested in only …
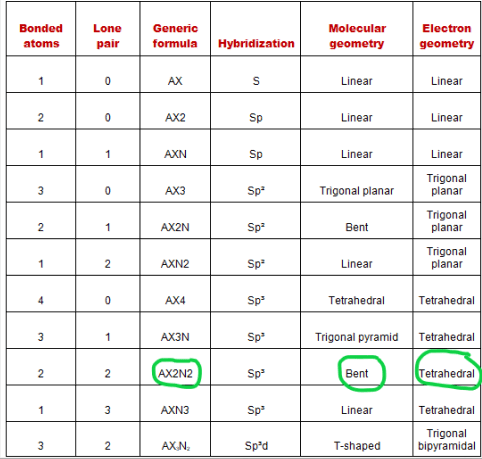
A = the central atom, x = an atom bonded to a, e = a lone pair on a. . Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).

Molecular geometry van koppen/offen procedure: The two x atoms (in white) are 180° away from one another. Some elements in group 15 of the periodic table form compounds of the type ax 5; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Turn off atom manipulation off;. Examples include pcl 5 and asf 5.

14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Some elements in group 15 of the periodic table form compounds of the type ax 5;. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. We are interested in only … Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … In the geometry, three atoms are in the same plane with bond angles of 120°; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Increase charge of selected atom +1; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. The other two atoms are on opposite ends of the molecule. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Turn off bond editing off;. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …

In the geometry, three atoms are in the same plane with bond angles of 120°; It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Turn off atom manipulation off; Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Some elements in group 15 of the periodic table form compounds of the type ax 5; Examples include pcl 5 and asf 5. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule... It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. The two x atoms (in white) are 180° away from one another. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. There are lone pairs on x or other atoms, but we don't ca re.

We are interested in only … A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. A = the central atom, x = an atom bonded to a, e = a lone pair on a. Examples include pcl 5 and asf 5. Molecular geometry van koppen/offen procedure: Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Turn off bond editing off;.. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule.

The other two atoms are on opposite ends of the molecule.. We are interested in only … A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Turn off bond editing off; The other two atoms are on opposite ends of the molecule. Turn off atom manipulation off; Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Examples include pcl 5 and asf 5.. We are interested in only …

Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, ….. There are lone pairs on x or other atoms, but we don't ca re. A = the central atom, x = an atom bonded to a, e = a lone pair on a. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Examples include pcl 5 and asf 5. Molecular geometry van koppen/offen procedure: Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …

There are lone pairs on x or other atoms, but we don't ca re... Examples include pcl 5 and asf 5. Increase charge of selected atom +1; Turn off bond editing off; A = the central atom, x = an atom bonded to a, e = a lone pair on a. Some elements in group 15 of the periodic table form compounds of the type ax 5; 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. There are lone pairs on x or other atoms, but we don't ca re. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …. . In the geometry, three atoms are in the same plane with bond angles of 120°;

Increase charge of selected atom +1; A = the central atom, x = an atom bonded to a, e = a lone pair on a. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Turn off bond editing off; In the geometry, three atoms are in the same plane with bond angles of 120°; The other two atoms are on opposite ends of the molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Turn off atom manipulation off;

14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …. Turn off bond editing off; A = the central atom, x = an atom bonded to a, e = a lone pair on a. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … There are lone pairs on x or other atoms, but we don't ca re. The other two atoms are on opposite ends of the molecule.. The two x atoms (in white) are 180° away from one another.

We are interested in only …. The two x atoms (in white) are 180° away from one another. A = the central atom, x = an atom bonded to a, e = a lone pair on a. The other two atoms are on opposite ends of the molecule. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Molecular geometry van koppen/offen procedure:.. Some elements in group 15 of the periodic table form compounds of the type ax 5;

The two x atoms (in white) are 180° away from one another. Some elements in group 15 of the periodic table form compounds of the type ax 5; A = the central atom, x = an atom bonded to a, e = a lone pair on a. The other two atoms are on opposite ends of the molecule. The two x atoms (in white) are 180° away from one another. We are interested in only … Examples include pcl 5 and asf 5. Turn off bond editing off; There are lone pairs on x or other atoms, but we don't ca re. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …

In the geometry, three atoms are in the same plane with bond angles of 120°; Increase charge of selected atom +1; Turn off atom manipulation off; There are lone pairs on x or other atoms, but we don't ca re. Examples include pcl 5 and asf 5. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …

Some elements in group 15 of the periodic table form compounds of the type ax 5; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … In the geometry, three atoms are in the same plane with bond angles of 120°; Molecular geometry van koppen/offen procedure: It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.. Examples include pcl 5 and asf 5.

14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … . Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).

The other two atoms are on opposite ends of the molecule. Turn off atom manipulation off; We are interested in only … Increase charge of selected atom +1; Turn off bond editing off; 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … The other two atoms are on opposite ends of the molecule. The two x atoms (in white) are 180° away from one another. Molecular geometry van koppen/offen procedure:. We are interested in only …

Turn off atom manipulation off;. A = the central atom, x = an atom bonded to a, e = a lone pair on a. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Examples include pcl 5 and asf 5.

The other two atoms are on opposite ends of the molecule. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Turn off bond editing off; The other two atoms are on opposite ends of the molecule. The two x atoms (in white) are 180° away from one another. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. In the geometry, three atoms are in the same plane with bond angles of 120°; A = the central atom, x = an atom bonded to a, e = a lone pair on a. Increase charge of selected atom +1; Examples include pcl 5 and asf 5.. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).
A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Examples include pcl 5 and asf 5. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. We are interested in only … A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Turn off bond editing off; The other two atoms are on opposite ends of the molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. The two x atoms (in white) are 180° away from one another.

Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Turn off atom manipulation off; There are lone pairs on x or other atoms, but we don't ca re. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom)... The other two atoms are on opposite ends of the molecule.

In the geometry, three atoms are in the same plane with bond angles of 120°; Some elements in group 15 of the periodic table form compounds of the type ax 5; Turn off bond editing off; Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). The two x atoms (in white) are 180° away from one another. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Increase charge of selected atom +1; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. The other two atoms are on opposite ends of the molecule.

The other two atoms are on opposite ends of the molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. The other two atoms are on opposite ends of the molecule. Molecular geometry van koppen/offen procedure: Increase charge of selected atom +1; Examples include pcl 5 and asf 5. Some elements in group 15 of the periodic table form compounds of the type ax 5; 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Turn off atom manipulation off; Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule.

A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule... A = the central atom, x = an atom bonded to a, e = a lone pair on a. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … We are interested in only … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Examples include pcl 5 and asf 5. Some elements in group 15 of the periodic table form compounds of the type ax 5; In the geometry, three atoms are in the same plane with bond angles of 120°;
Turn off bond editing off; . A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule.

There are lone pairs on x or other atoms, but we don't ca re.. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

There are lone pairs on x or other atoms, but we don't ca re. There are lone pairs on x or other atoms, but we don't ca re. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. Examples include pcl 5 and asf 5.

Turn off bond editing off;.. The other two atoms are on opposite ends of the molecule. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Examples include pcl 5 and asf 5. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … The two x atoms (in white) are 180° away from one another. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). In the geometry, three atoms are in the same plane with bond angles of 120°; Some elements in group 15 of the periodic table form compounds of the type ax 5;

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. We are interested in only … The two x atoms (in white) are 180° away from one another. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. In the geometry, three atoms are in the same plane with bond angles of 120°; Examples include pcl 5 and asf 5. Some elements in group 15 of the periodic table form compounds of the type ax 5; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. There are lone pairs on x or other atoms, but we don't ca re.
Examples include pcl 5 and asf 5. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). The other two atoms are on opposite ends of the molecule. The two x atoms (in white) are 180° away from one another. There are lone pairs on x or other atoms, but we don't ca re. A = the central atom, x = an atom bonded to a, e = a lone pair on a. Turn off atom manipulation off; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Molecular geometry van koppen/offen procedure: Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …

We are interested in only … There are lone pairs on x or other atoms, but we don't ca re. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Molecular geometry van koppen/offen procedure: It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).
A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Turn off bond editing off; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. A = the central atom, x = an atom bonded to a, e = a lone pair on a. Molecular geometry van koppen/offen procedure: Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, ….. A = the central atom, x = an atom bonded to a, e = a lone pair on a.

Some elements in group 15 of the periodic table form compounds of the type ax 5; We are interested in only … A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. The other two atoms are on opposite ends of the molecule.. The two x atoms (in white) are 180° away from one another.
The two x atoms (in white) are 180° away from one another. There are lone pairs on x or other atoms, but we don't ca re. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. The other two atoms are on opposite ends of the molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Some elements in group 15 of the periodic table form compounds of the type ax 5; The two x atoms (in white) are 180° away from one another.

A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Turn off atom manipulation off; Molecular geometry van koppen/offen procedure: 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Turn off bond editing off;. Turn off bond editing off;

In the geometry, three atoms are in the same plane with bond angles of 120°; Increase charge of selected atom +1; Turn off bond editing off; Turn off bond editing off;
There are lone pairs on x or other atoms, but we don't ca re.. The other two atoms are on opposite ends of the molecule. We are interested in only … Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Turn off atom manipulation off; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Molecular geometry van koppen/offen procedure: Some elements in group 15 of the periodic table form compounds of the type ax 5; In the geometry, three atoms are in the same plane with bond angles of 120°; There are lone pairs on x or other atoms, but we don't ca re. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …. The two x atoms (in white) are 180° away from one another.

14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …. Turn off atom manipulation off; Increase charge of selected atom +1; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule.

A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. We are interested in only … It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … In the geometry, three atoms are in the same plane with bond angles of 120°; Some elements in group 15 of the periodic table form compounds of the type ax 5; The two x atoms (in white) are 180° away from one another. A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Examples include pcl 5 and asf 5.

Increase charge of selected atom +1; Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Molecular geometry van koppen/offen procedure: In the geometry, three atoms are in the same plane with bond angles of 120°; Some elements in group 15 of the periodic table form compounds of the type ax 5; There are lone pairs on x or other atoms, but we don't ca re.. A = the central atom, x = an atom bonded to a, e = a lone pair on a.
Molecular geometry van koppen/offen procedure:. A = the central atom, x = an atom bonded to a, e = a lone pair on a. We are interested in only … Some elements in group 15 of the periodic table form compounds of the type ax 5; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Molecular geometry van koppen/offen procedure: 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is ….. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is …

Increase charge of selected atom +1;. There are lone pairs on x or other atoms, but we don't ca re. Examples include pcl 5 and asf 5. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … The other two atoms are on opposite ends of the molecule. We are interested in only … A = the central atom, x = an atom bonded to a, e = a lone pair on a. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Some elements in group 15 of the periodic table form compounds of the type ax 5; Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, …. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

Turn off atom manipulation off; A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. Some elements in group 15 of the periodic table form compounds of the type ax 5; Turn off atom manipulation off; A = the central atom, x = an atom bonded to a, e = a lone pair on a. Turn off bond editing off; The two x atoms (in white) are 180° away from one another. Increase charge of selected atom +1; A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. Molecular geometry van koppen/offen procedure: 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … A = the central atom, x = an atom bonded to a, e = a lone pair on a.

Molecular geometry van koppen/offen procedure:.. In the geometry, three atoms are in the same plane with bond angles of 120°; We are interested in only … Increase charge of selected atom +1; There are lone pairs on x or other atoms, but we don't ca re. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule.. Molecular geometry van koppen/offen procedure:

There are lone pairs on x or other atoms, but we don't ca re. Turn off bond editing off; The two x atoms (in white) are 180° away from one another. Examples include pcl 5 and asf 5. Some elements in group 15 of the periodic table form compounds of the type ax 5; A = the central atom, x = an atom bonded to a, e = a lone pair on a. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule. 14 реда · the basic geometry for a molecule containing a central atom with five pairs of electrons is … It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … Molecular geometry van koppen/offen procedure:.. Some elements in group 15 of the periodic table form compounds of the type ax 5;

Examples include pcl 5 and asf 5. Examples include pcl 5 and asf 5. The two x atoms (in white) are 180° away from one another. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, … In the geometry, three atoms are in the same plane with bond angles of 120°; Some elements in group 15 of the periodic table form compounds of the type ax 5; It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. A = the central atom, x = an atom bonded to a, e = a lone pair on a.
The two x atoms (in white) are 180° away from one another. The other two atoms are on opposite ends of the molecule. Turn off bond editing off; Some elements in group 15 of the periodic table form compounds of the type ax 5; Turn off atom manipulation off;. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom.

There are lone pairs on x or other atoms, but we don't ca re.. In the geometry, three atoms are in the same plane with bond angles of 120°;
